About Profound

INTEGRATED SITE OPERATION

FAST ACTIVATION

RELIABLE ENROLLMENT
ENHANCED STUDY CONDUCT
PROFOUND'S ENGAGEMENT MODEL
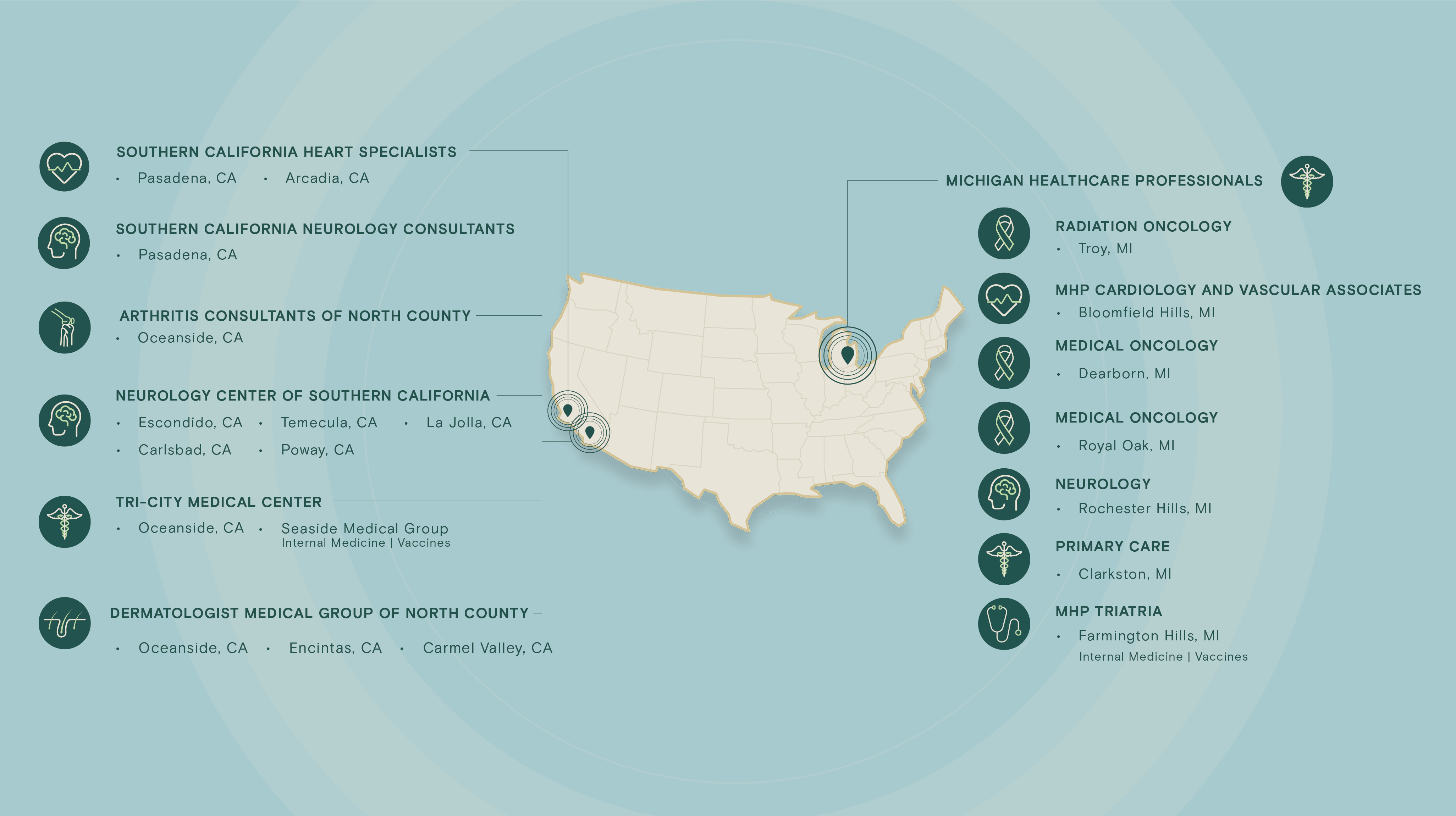
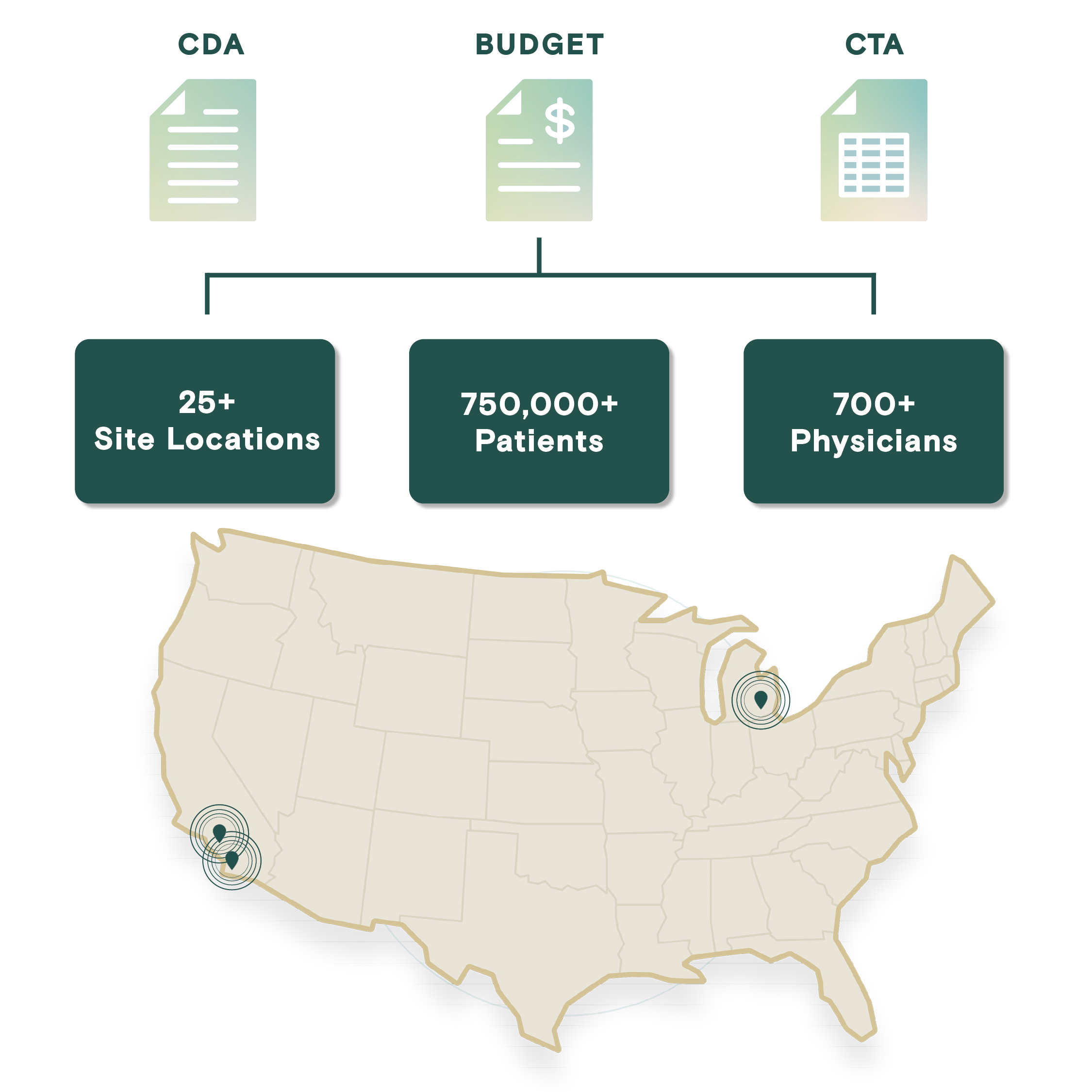
Profound Research presents an integrated site network comprising over 25 sites with an extensive roster of 700+ physicians, including numerous board-certified specialists. This network grants access to a diverse and expansive pool of over 750,000 patients, spanning a comprehensive spectrum of therapeutic areas such as neurology, dermatology, rheumatology, oncology, cardiovascular, primary care, endocrinology, and vaccines.
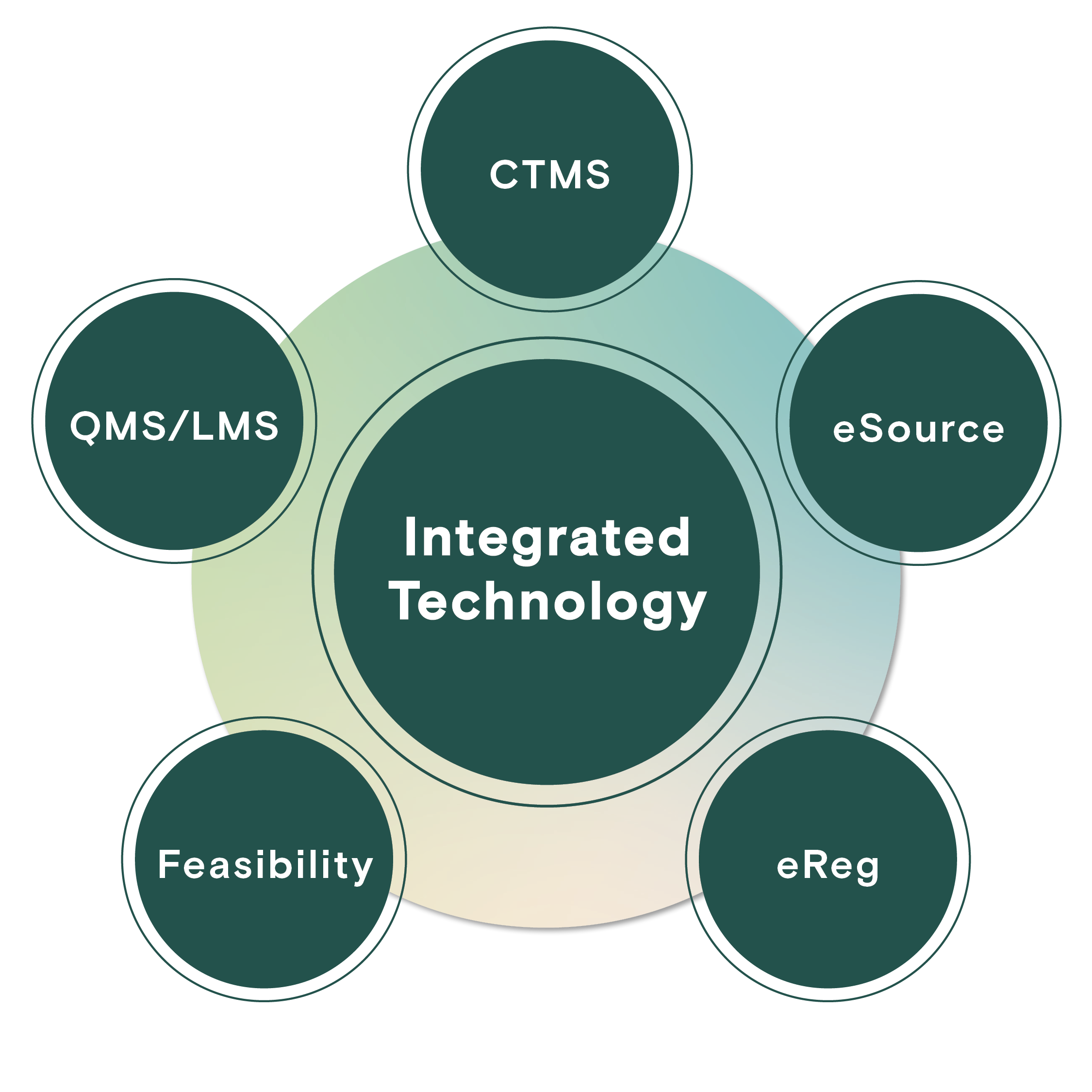
Profound provides our sites a centralized hub for key activities crucial to efficient feasibility, budgeting, contracting, quality assurance, regulatory compliance, and study management. These essential functions are supported by a sophisticated technology stack that incorporates CTMS, eReg, eSource, QMS/LMS, and robust training modules. This technological foundation underscores our unwavering commitment to patient safety, principal investigator oversight, and the delivery of high-quality data.
Therapeutic Areas
Select a therapeutic area below to explore sites:
Full-Service Tech Stack
Profound Research provides a best-in-class suite of technology solutions which enable access, transparency, standardization, and efficient processes across our network.
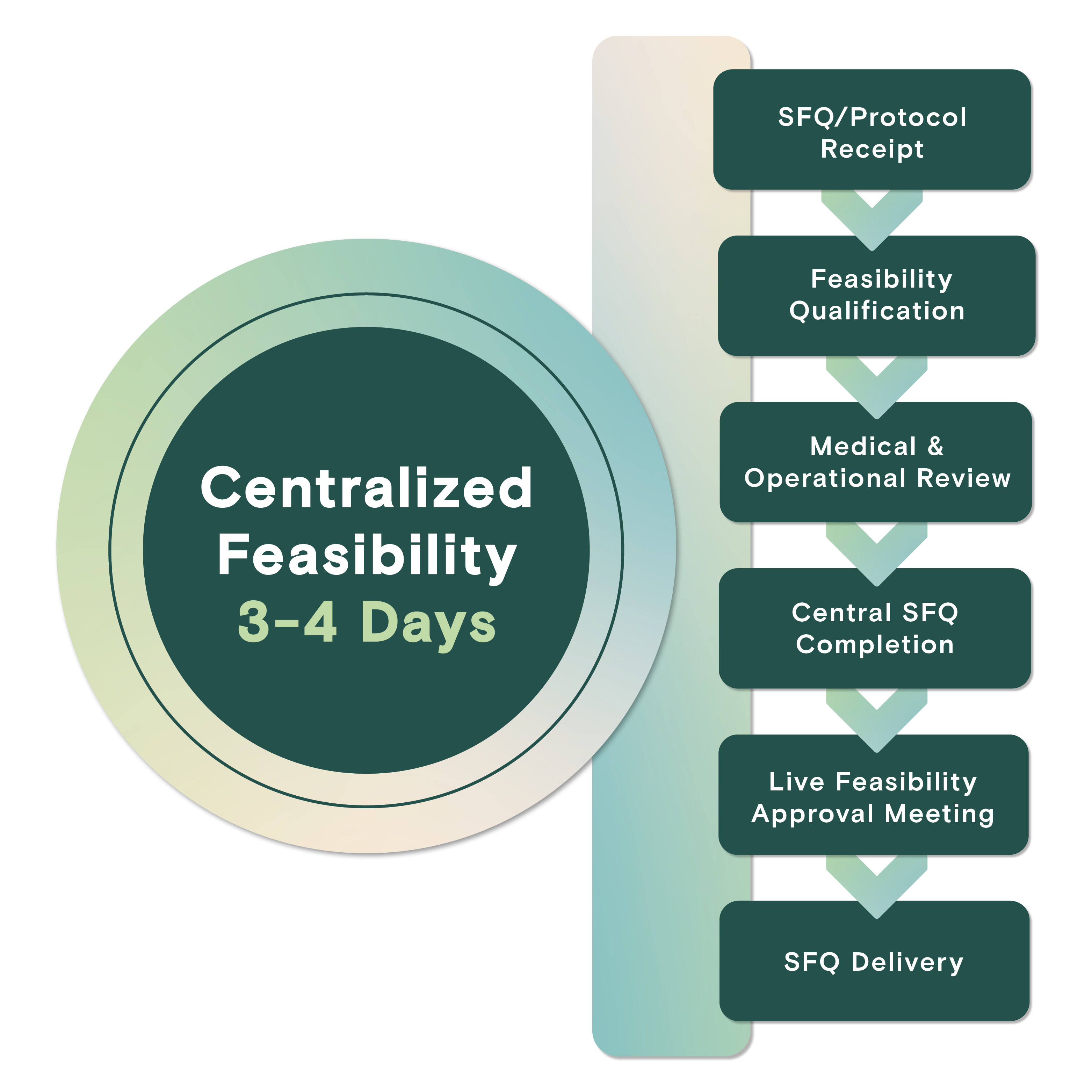
Centralized Feasibility
Dedicated resources facilitate detailed review of study protocols to provide data-driven enrollment commitments through centralized EMR access and streamlined site communication, resulting in industry-leading turnaround timelines.
Centralized Budget and Contracting
Profound reduces friction and delays in budget and CTA execution through dedicated resources, robust processes, and a commitment to accelerating timelines while maintaining quality.
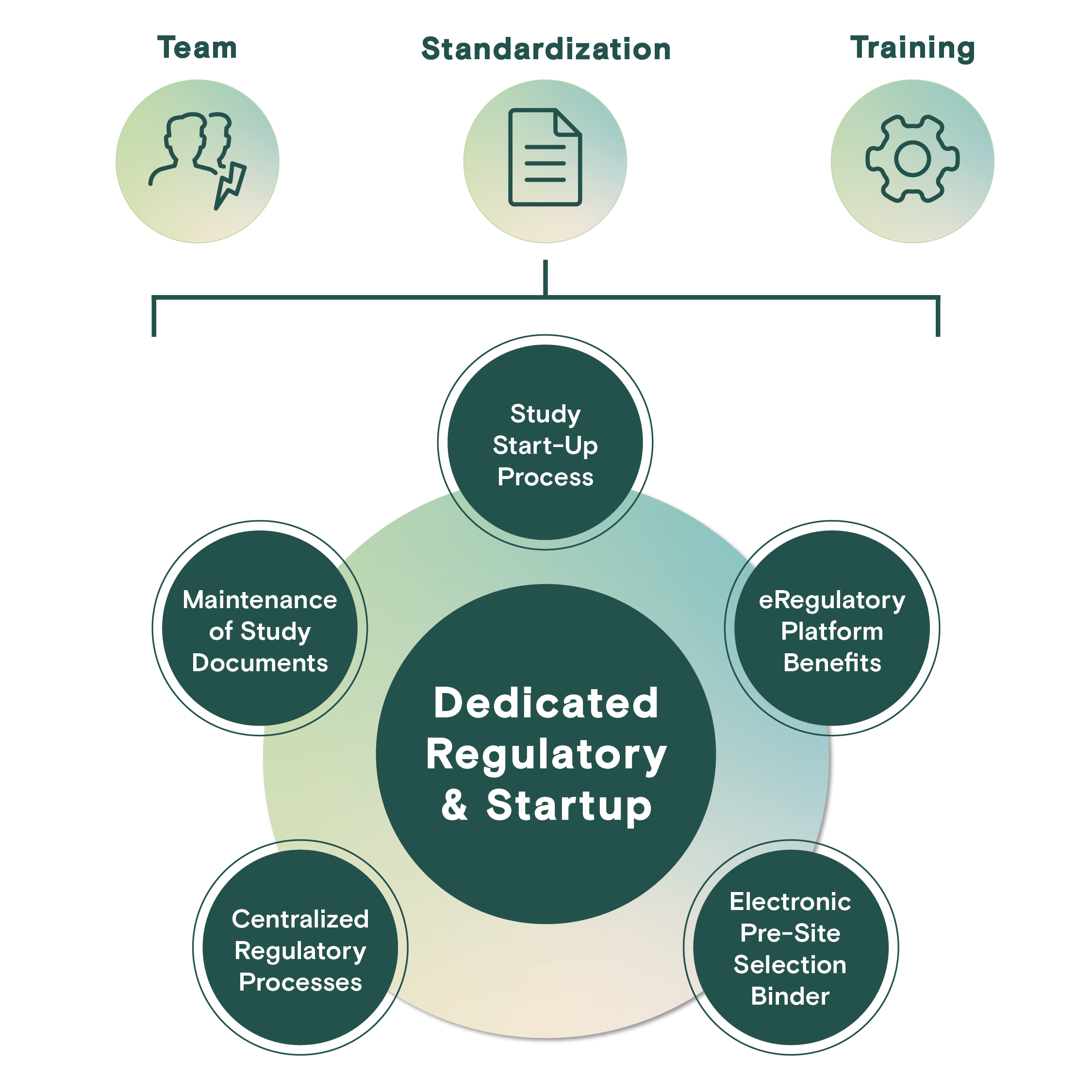
Dedicated Regulatory & Start-Up
Profound's eRegulatory platform and centralized regulatory team manages study start-up activities resulting in expedited study initiation while reducing site burden during this critical time in the study lifecycle.
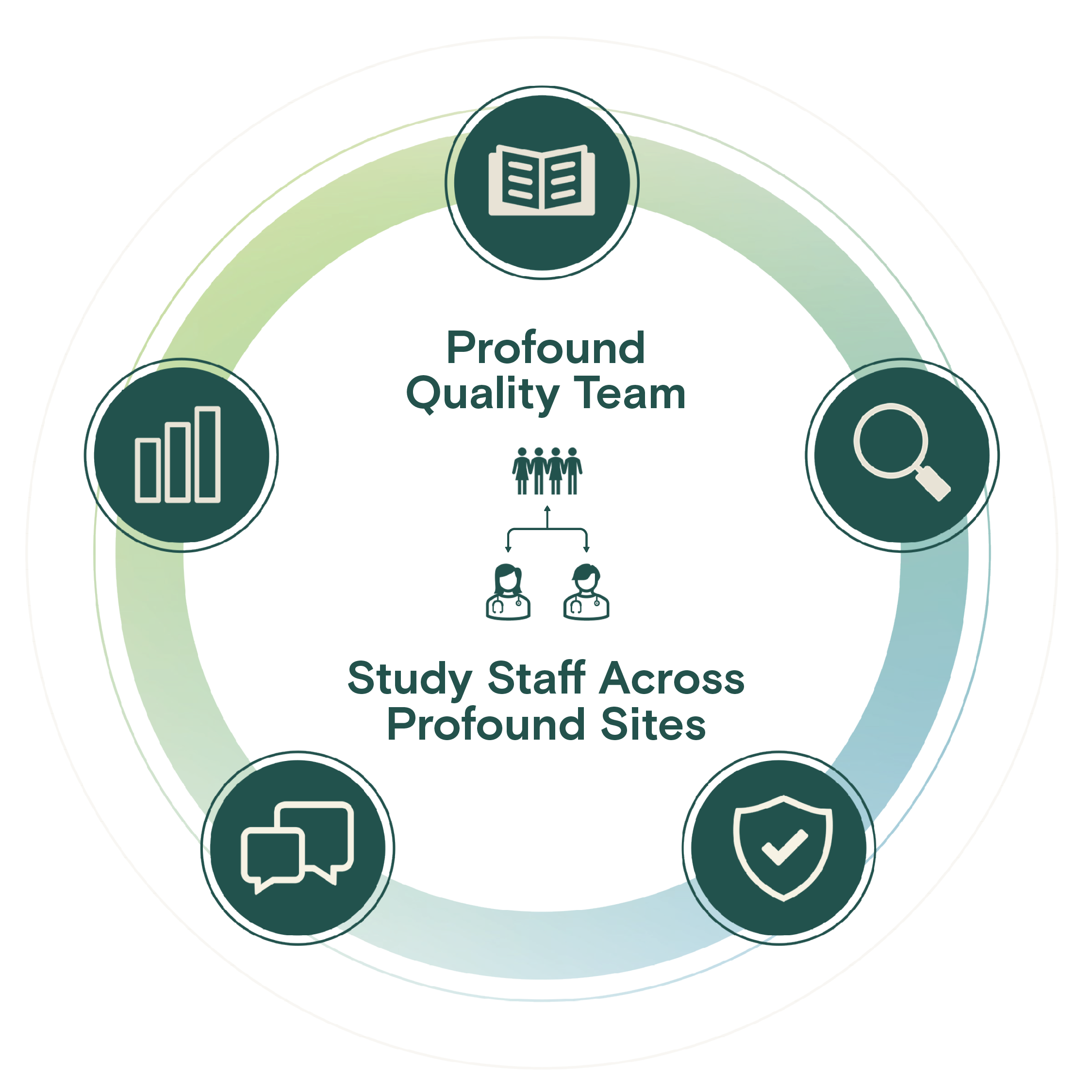
Centralized Quality, Compliance, & Inspection Readiness
Our quality team leverages an industry-leading quality management system to deploy training, processes, and internal auditing within a continuous process improvement culture.